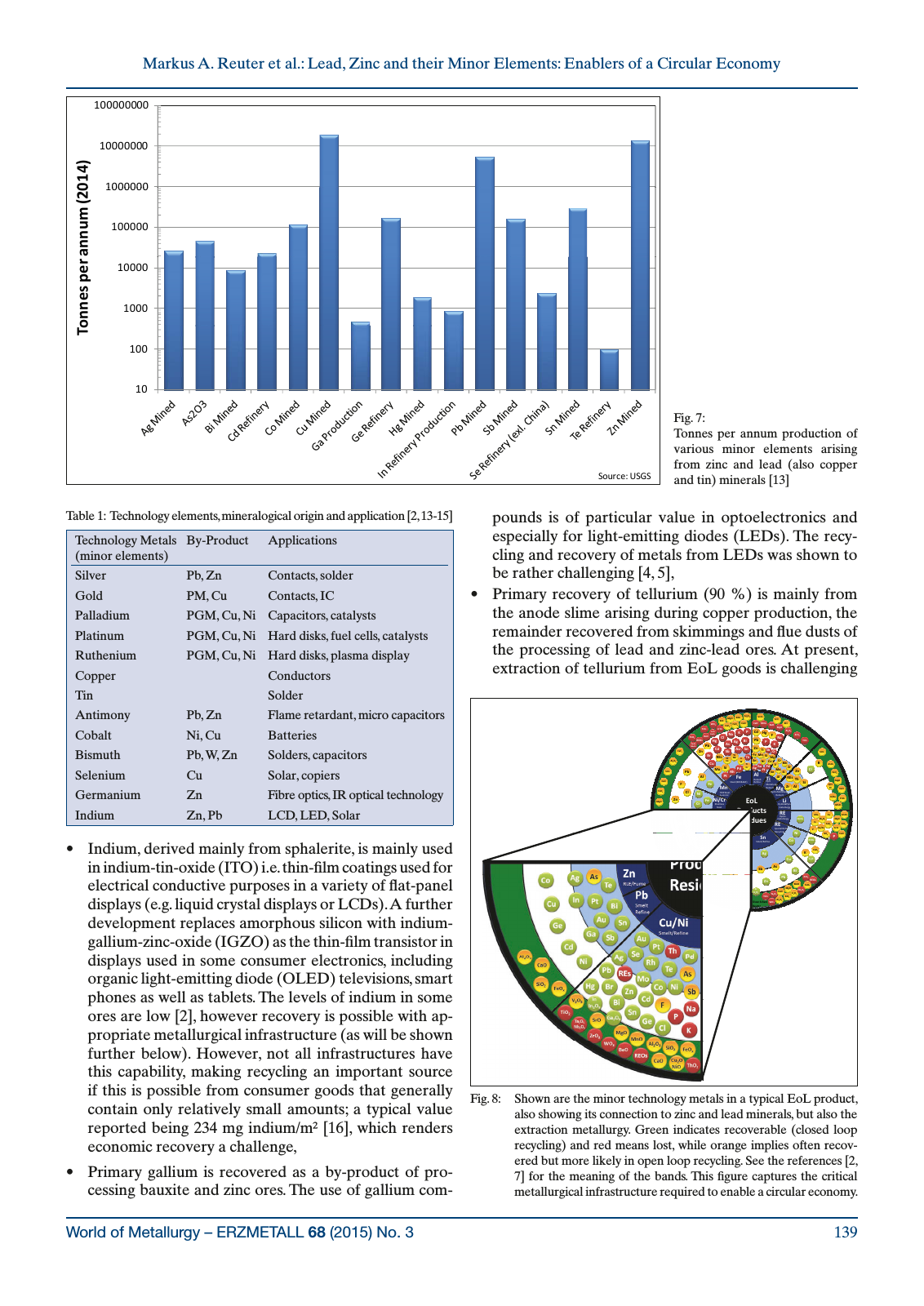
World of Metallurgy ERZMETALL 68 2015 No 3 139 Markus A Reuter et al Lead Zinc and their Minor Elements Enablers of a Circular Economy Indium derived mainly from sphalerite is mainly used in indium tin oxide ITO i e thin film coatings used for electrical conductive purposes in a variety of flat panel displays e g liquid crystal displays or LCDs A further development replaces amorphous silicon with indium gallium zinc oxide IGZO as the thin film transistor in displays used in some consumer electronics including organic light emitting diode OLED televisions smart phones as well as tablets The levels of indium in some ores are low 2 however recovery is possible with ap propriate metallurgical infrastructure as will be shown further below However not all infrastructures have this capability making recycling an important source if this is possible from consumer goods that generally contain only relatively small amounts a typical value reported being 234 mg indium m 16 which renders economic recovery a challenge Primary gallium is recovered as a by product of pro cessing bauxite and zinc ores The use of gallium com pounds is of particular value in optoelectronics and especially for light emitting diodes LEDs The recy cling and recovery of metals from LEDs was shown to be rather challenging 4 5 Primary recovery of tellurium 90 is mainly from the anode slime arising during copper production the remainder recovered from skimmings and flue dusts of the processing of lead and zinc lead ores At present extraction of tellurium from EoL goods is challenging Fig 7 Tonnes per annum production of various minor elements arising from zinc and lead also copper and tin minerals 13 Figure 7 Reuter 100000000 1000000 10000000 014 100000 an nu m 2 0 1000 10000 on ne s pe r 10 100 To Source USGS Technology Metals minor elements By Product Applications Silver Pb Zn Contacts solder Gold PM Cu Contacts IC Palladium PGM Cu Ni Capacitors catalysts Platinum PGM Cu Ni Hard disks fuel cells catalysts Ruthenium PGM Cu Ni Hard disks plasma display Copper Conductors Tin Solder Antimony Pb Zn Flame retardant micro capacitors Cobalt Ni Cu Batteries Bismuth Pb W Zn Solders capacitors Selenium Cu Solar copiers Germanium Zn Fibre optics IR optical technology Indium Zn Pb LCD LED Solar Table 1 Technology elements mineralogical origin and application 2 13 15 Au Sn SiO2 Ag Cd REOs Sb Al2O3 Al2O3 Cl As Ti Na KP Na K P TiO2 MgO ZrO2 BaO TiO2 ZrO2 BaO REOs WO3 CaO MgO Ta2O5 Nb2O5 FeOx Bi Pb Hg Pb Hg Zn CaO Cd ThO2 In2O3 Ga2O3 SrO V2O5 SrO F KCl CaF2 Mn Fe Steel BOF EAF Al Remelt Refine Sb Pt Pd Cu Pd Pt AuAg Cr Cu SiBr Fe NiSn MnREs Mo Ta Nb Rh Si Zn Mg SiO2 Al2O3 CaO FeO Th Pb F Cl CaF2 SiO2 CaO Al2O3 MgO F Cl Br FeOx B ClZn AlMg H d M l l Si REs Zr Th Fe Al Ti Pyro Metal lurgy Remelt Si Al Sn Mo ZrV NbCr Ni Co V W Ti Cr Zn RLE Fume TRIP Steel Austenitic Ni Cr Stain less Steel RE Hy dro meta l lurgy Bi Ni Fe CrMgO x In Co Cu Pt Pb Smelt Refine RE O s SiO2 Al2O3 SrO MgOSb2O3 EoL Products Residues RE Special Battery Recy clin g Ni Zn CaXy AlXy Cu Ga AsP In K Na MnO P2O5Li H y dro Pyro metallurgy H y d ro M eta l l urgy Remelt Ag As Te Cu Ni Smelt Refine Br CdBi Au Ag Pd Pt REs Sn Sb Pb ZnHg Mo Co Rh As Sb SiO2 CaO Al2O3 FeOx Ge Cd Te Se Au f n Ga P CaO Th y g Co K FeOx REOs V2O5 In Sn Smelt Refine SiXy Li2O Bi Ag Cu AsSb Ni Ni van Schaik Reuter Cl Sn Ge TiO2 ZrO2 BaO P Na K REOs WO3 SiO2 CaO Al2O3 Cu2O NiO FeOx MgO MnO Ga2O3 ThO2 SrO FIn2O3 Ta2O5 Pb Zn TiO2 ZrO2 REOs WO3 SiO2 CaO Al2O3 FeOx MgO MnO In In2O3 V2O5 Nb2O5 Ta2O5 Nb2O5 Fig 8 Shown are the minor technology metals in a typical EoL product also showing its connection to zinc and lead minerals but also the extraction metallurgy Green indicates recoverable closed loop recycling and red means lost while orange implies often recov ered but more likely in open loop recycling See the references 2 7 for the meaning of the bands This figure captures the critical metallurgical infrastructure required to enable a circular economy
Hinweis: Dies ist eine maschinenlesbare No-Flash Ansicht.
Klicken Sie hier um zur Online-Version zu gelangen.
Klicken Sie hier um zur Online-Version zu gelangen.